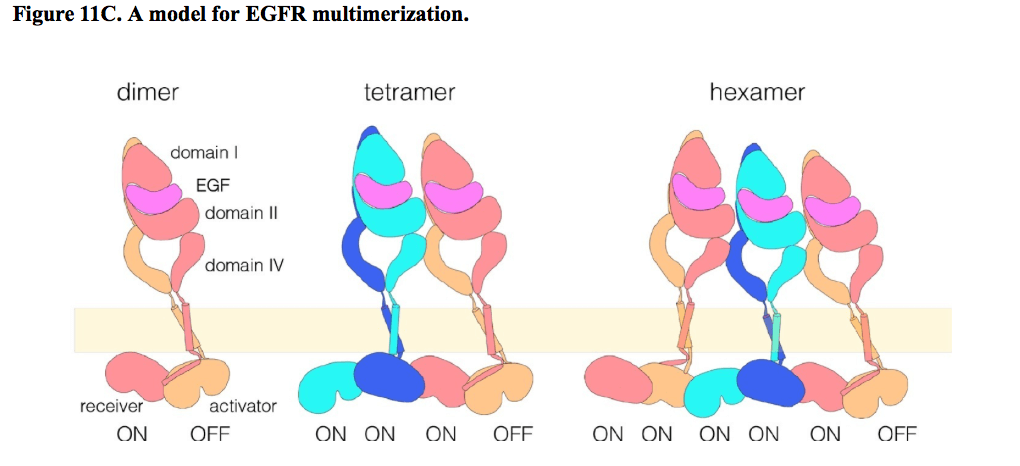
Molecular Basis for Multimerization in the Activation of the Epidermal Growth Factor Receptor
Huang et al. eLife, 5:e14107. (2016) DOI: 10.7554/eLife.14107
Reviewed by Yuliya Birman, Biophysics Graduate Program, Arkin lab, yuliya.birman@ucsf.edu
The epidermal growth factor receptor (EGFR) is a cell surface receptor that plays an important role in signaling pathways. Aberrant EGFR-mediated signaling is associated with cancer, and there is great interest in understanding how these receptors are regulated. EGFR is activated by dimerization, but activation also generates higher-order oligomers (“multimers”). The nature and importance of these multimers remained poorly understood until now. Here, Huang, et. al. used single-molecule imaging, mutational analysis, and computational modeling to identify a region in EGFR that mediates multimerization. This region was found to be important for the function of EGFR and associated signaling kinases. Using this information, the authors proposed a structural model for EGFR multimerization. Although their model is preliminary and raises many questions on the mechanism of EGFR oligomerization and the importance of their identified multimerization region, their elegantly-designed experiments have made a major contribution to our basic understanding of EGFR signaling.
Yuliya is a biophysics grad student in the Arkin lab who is studying how cells maintain protein quality and quantity in normal vs. disease states.